Founded in June 2015 as a wholly owned subsidiary of Highnoon Laboratories Ltd. Curexa Health has grown into a cGMP-certified, state-of-the-art manufacturer of cephalosporin-based antibiotics. Beginning operations in 2017 with sterile injectables and expanding to Oral Solid Dosage production in 2019, we now serve both local and international markets. Our commitment to quality is reinforced by global certifications, including cGMP (DRAP), ISO 9001, ISO/IEC 17025, ISO 14001, and ISO 45001. In
2024, Curexa evolved into a standalone vertical business entity, focused on delivering trusted healthcare solutions through advanced science, innovation, and uncompromising quality.
Our Mission & Vision
Mission
Mission
Curexa’s mission is to transform authentic craft into accessible, high-quality Primary & Acute Healthcare therapies that improve patients’ lives every day.
Vision
Vision
Curexa envisions bridging craft and health to set a global benchmark for authentic patient impact.
Purpose
Purpose
To bridge our craft to health, delivering authentic, high-quality Primary & Acute Healthcare therapies that improve lives
Our Core Values
Integrity
Integrity
We act with honesty, ethics and responsibility in all we do.
Meritocracy & Excellence
Meritocracy & Excellence
We recognize talent, reward performance and strive for the highest standards.
Patience Centricity
Patience Centricity
Every decision and
action begins with the patient at the heart.
Agility & Innovation
Agility & Innovation
We adapt fast,
embrace creativity, and drive foward-thinking
solutions.
Collaboration & Partnerships
Collaboration & Partnerships
We work together and build strong alliances for collective success.
Total Quality (Relentless)
Total Quality (Relentless)
We ensure uncompromising, consistent quality across everything we deliver.
CUREXA BOARD OF DIRECTORS
Designated and appointed under the authority of the Highnoon Board of Directors.

Mr Tausif Ahmad Khan

Mr Taufiq Ahmad Khan
Our Quality Policy
At Curexa Health, quality is at the core of everything we do.
We are dedicated to manufacturing safe, effective, and reliable products
that fully comply with current GMP standards, Quality Management
Systems, and all applicable regulatory requirements.
Our unwavering commitment to quality is sustained through:
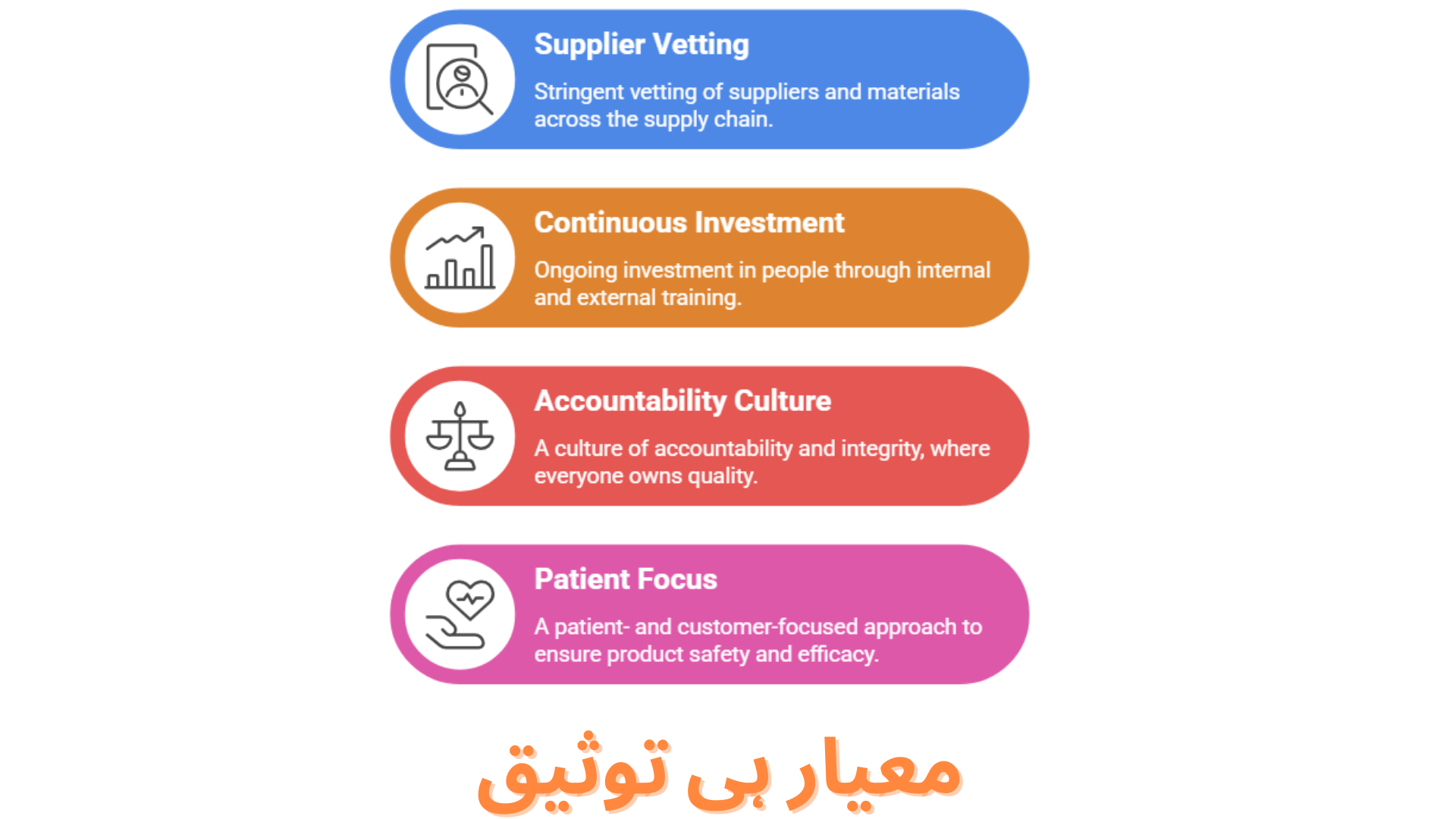
- Stringent supplier and material vetting across every stage of our supply chain.
- Continuous investment in people, empowering our teams through ongoing internal and external training programs.
- A culture of accountability and integrity, where every individual takes ownership of quality.
- A patient- and customer-focused approach, ensuring our products consistently meet the highest standards of safety and efficacy.
- At Curexa, quality isn’t just a process, it’s our promise.
Our EHS POLICY
ENVIRONMENTAL HEALTH & SAFETY POLICY
Curexa Health (Pvt.) Limited regards the Environmental matter along with Occupational Health & Safety as one of our priority issues, therefore company has created and implemented Environmental Health & Safety Management System (EHS). This System will be implemented, maintained and continually improved in accordance with ISO 14001:2015 and ISO 45001:2018 standards and will comply with legal and order requirements. standards and will comply with legal and order requirements.
This means that we will be:
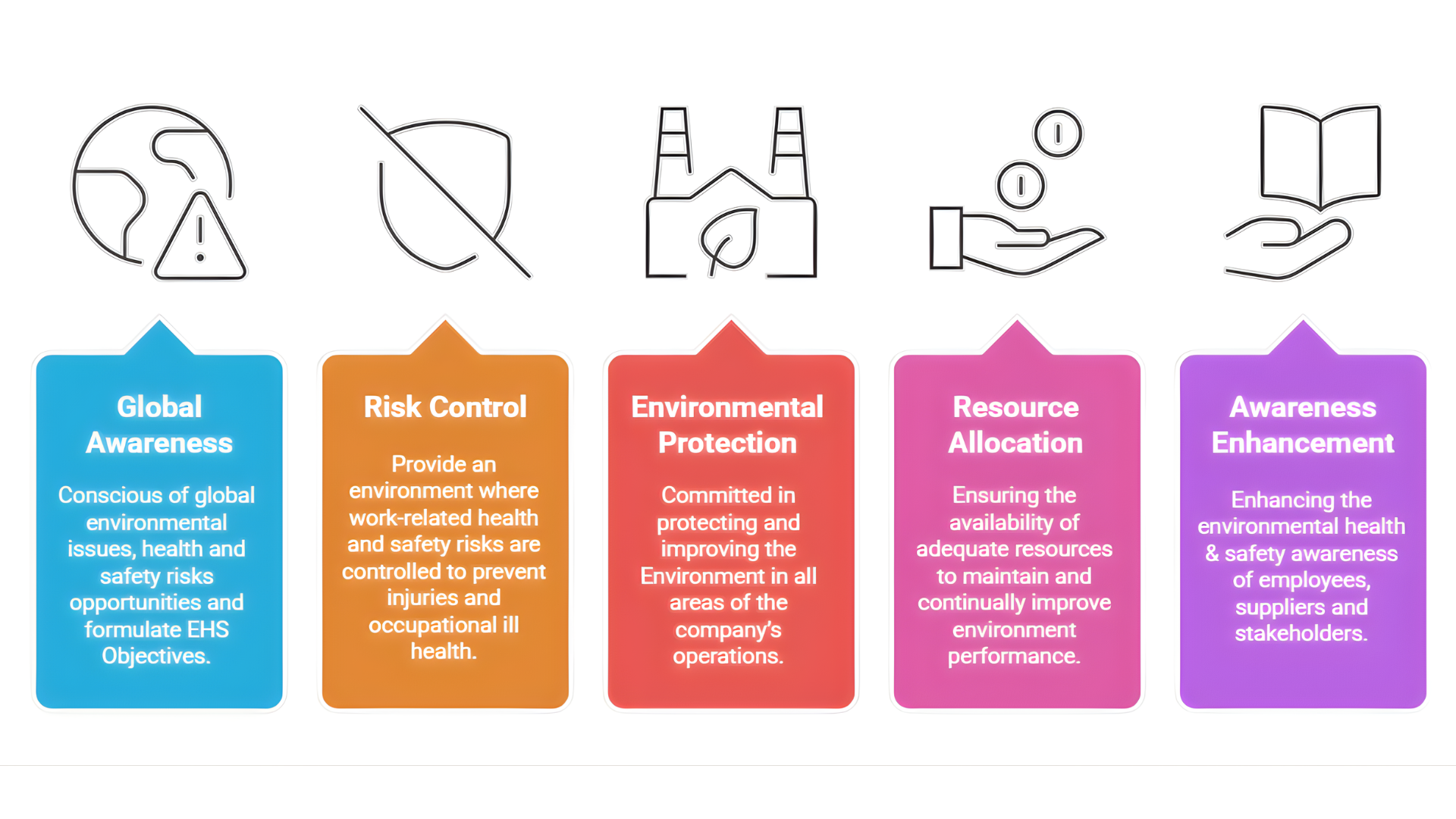
- Conscious of Global Environmental Issues, health and safety risks opportunities and formulate EHS Objectives
- Provide an environment where work-related health and safety risks are controlled to prevent injuries and occupational ill health.
- Committed in protecting and improving the Environment in all areas of the company’s operations, while simultaneously preserving and enhancing the Quality of life for employees, Customers, Neighbors and Community.
- Ensuring the availability of adequate resources to maintain and continually improve environment performance and control EHS risks and opportunities.
- Enhancing the environmental health & safety awareness of employees, suppliers and stakeholders through education and training to enhance environmental and social sustainability.
