Manufacturing Facility
Production at Curexa stands as the driving force of precision, performance, and purpose. Guided by our mission, we transform scientific expertise and craftsmanship into high-quality therapies that meet real-world healthcare needs. Our teams operate with unwavering passion, discipline, and innovation; ensuring that every process upholds the highest standards of quality, safety, and efficiency.
We apply advanced technology, validated processes, and qualified personnel to deliver products that meet global quality standards.
Our Formulation Capabilities
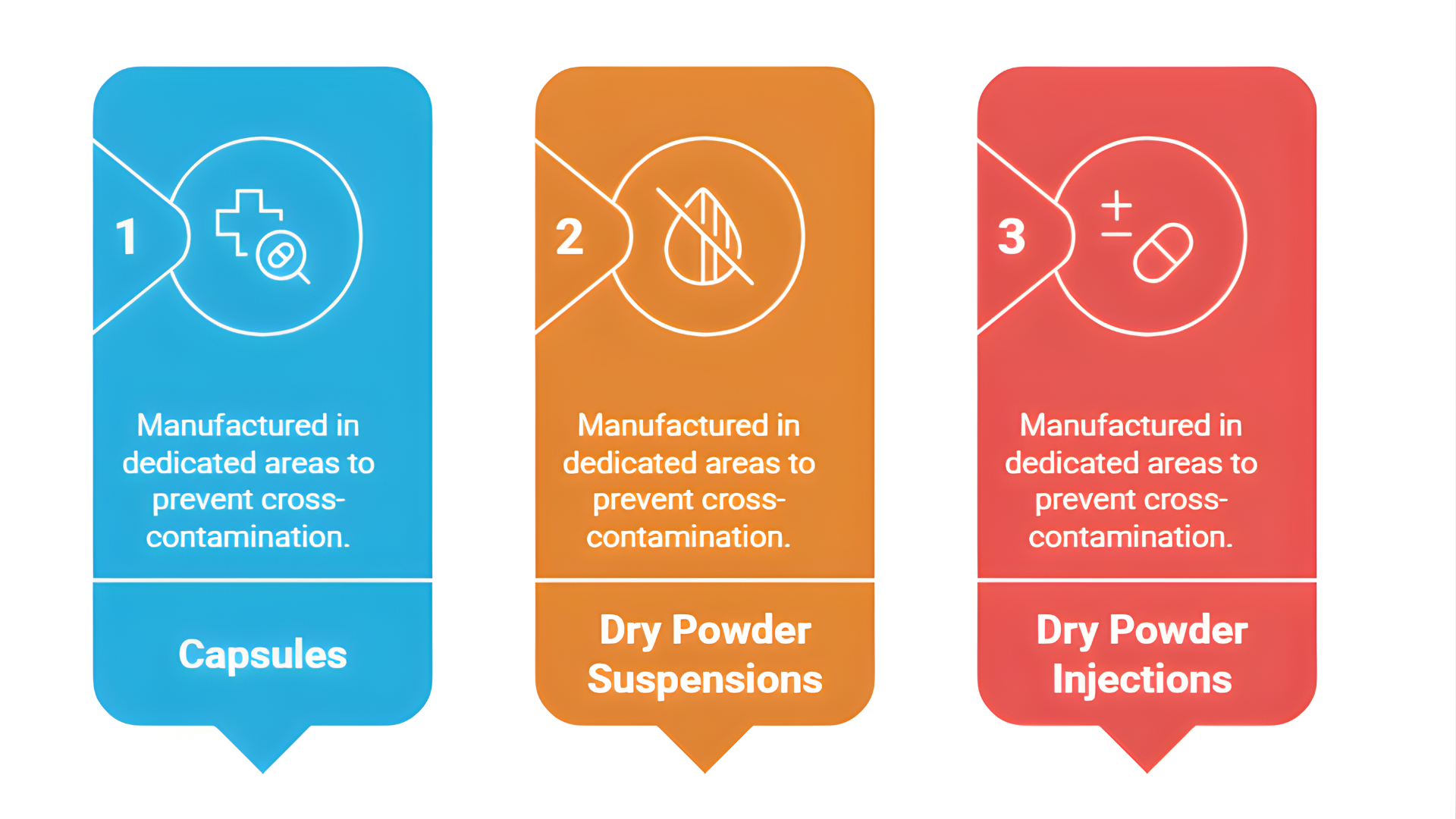
We are specialized in three cephalosporin dosage forms, each manufactured in dedicated areas to prevent cross-contamination:
- Capsules
- Dry Powder Suspensions
- Dry Powder Injections
Each production area is supported by dedicated HVAC systems, controlled personnel and material flows, and robust contamination-control protocols. All manufacturing operations are carried out on qualified equipment with validated processes. This ensures consistent product quality, uncompromised safety, and reliable therapeutic efficacy.
Technology That Sets Us Apart

At Curexa Health, our production operations leverage automation and stringent environmental controls to ensure uncompromised product quality. Fully automated lines, from vial washing and filling to sealing and labeling; maintain aseptic integrity at every stage. Our facility operates under validated sterile conditions, supported by HEPA filtration, controlled pressure differentials, and validated sterilization cycles, providing a consistently contamination-free environment.

To remain aligned with global pharmaceutical standards, we continuously upgrade our infrastructure, equipment, and processes. All aseptic manufacturing lines undergo routine Media Fill Trials (Aseptic Process Simulations) in accordance with international regulatory requirements, ensuring that every sterile batch meets the highest benchmarks for safety, sterility, and reproducibility.
Workforce & Training
Workforce & Training
Commitment to Excellence
Commitment to Excellence
Quality Operations
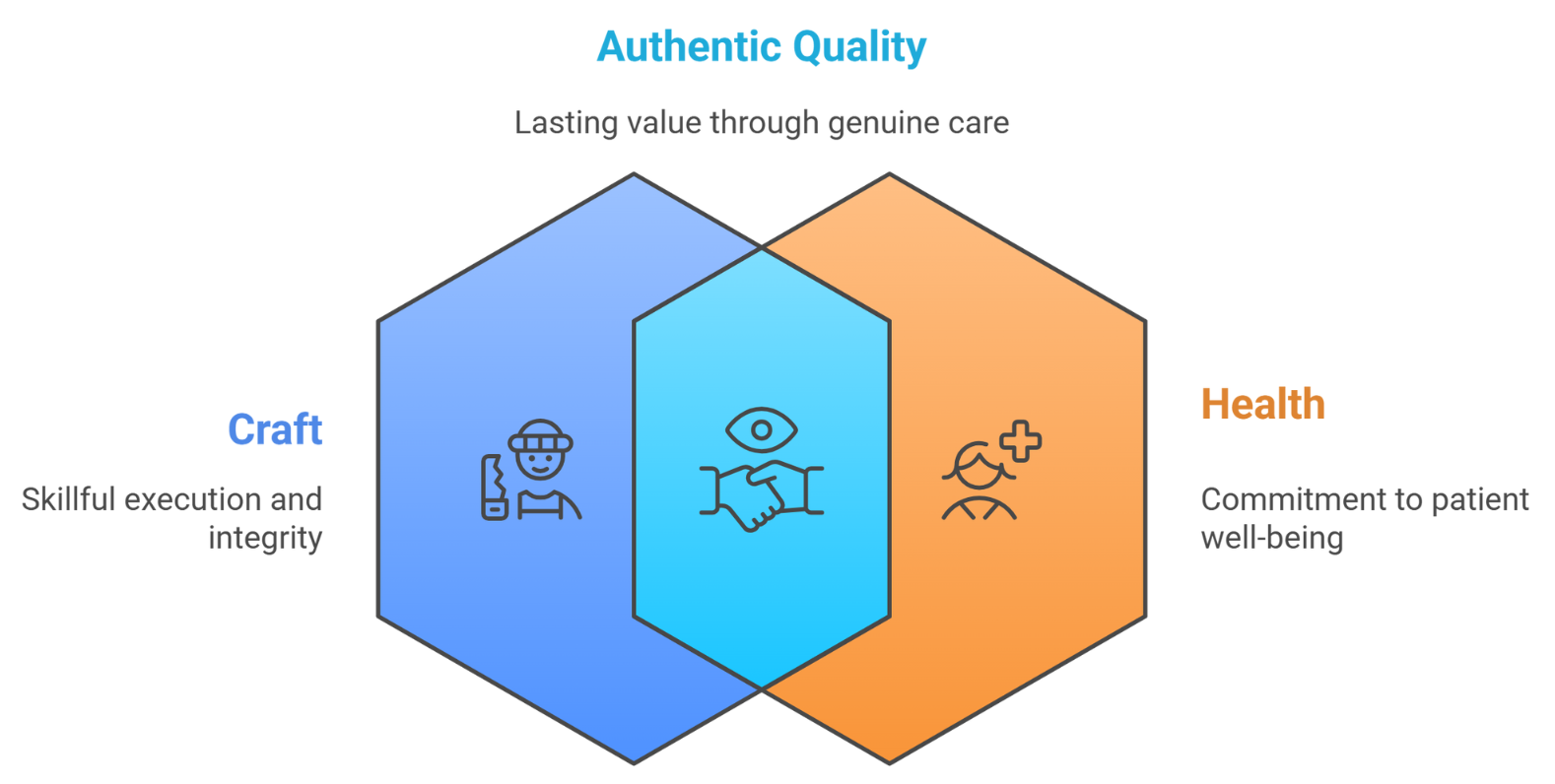
At Curexa Health, we make a solemn promise to bridge Craft (C) with Health (H) through Authentic Quality.
Quality is not a checkbox – it is our truthfulness to patients, healthcare professionals, regulators, and ourselves. It reflects our integrity, our craftsmanship, and our dedication to health. A collective promise to authentically connect Craft → Health, ensuring every act reflects Authenticity, Integrity, and Impact.
We believe Quality Crafted = Impact Delivered.
By embedding quality into every decision, we deliver not just compliance, but lasting value. Because when quality is authentic, it doesn’t just meet standards – it sets them.
